
Klasia Vision

Our Brand
CEO Greetings
KLASIA has developed competitive products and commercially viable items through creative R&D planning and execution.
In the future, we will optimize our execution and planning capabilities at all stages of drug development, enhance our R&D capabilities to create competitive business opportunities, and strive to contribute to the health of humanity and the development of the pharmaceutical industry.
We will also strive to improve the business environment in the pharmaceutical industry by correcting some of the wrong business practices and ensuring that competent and hardworking companies are recognized.
We will continue to work hard to be remembered as a company that develops good products and is trusted by all domestic and overseas companies and people with whom we have a stake.
 President James Chun
President James Chun
Organization

Certificates
Certificates

Certificate of Excellent Technology Evaluation Company
Development of Medicines to Acquire Exclusivity

Certificate of Technical Achievement
IMD Research and Development Technology

INNOBIZ COMPANY

Certificate of Technology Inno-Biz

Certificate of Inno-Biz(EN)

Certificate of Venture Enterprise

Certificate of Venture Enterprise(EN)

Certificate of Department in Charge of R&D

Certificate of Authorization(EN)
Certificate of Patent

Certificate of Patent 10-2216470

Certificate of Patent 10-2481517
Licence

Drug Wholesale Dealer licence

Registration Document of The Import Business
Membership Cards

Certificate of Membership (Korea International Trade Association)
Award

The marquis who's who(EN)
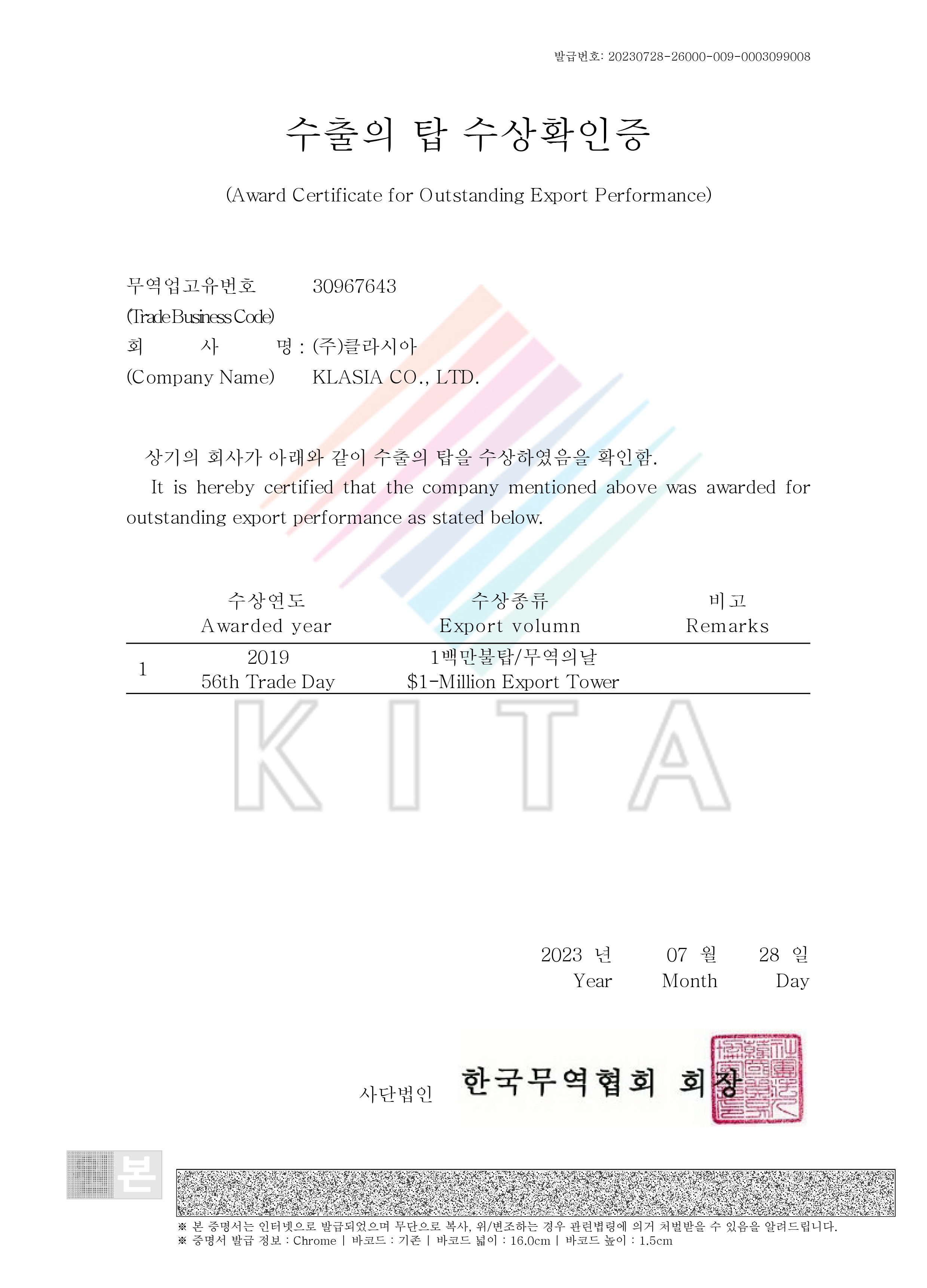
Award Certificate for Outstanding Export Performance ($1-Million Export Tower)

Award Plaque for Outstanding Export Performance ($1-Million Export Tower)

Award Certificate for Outstanding Export Performance ($3-Million Export Tower)

Award Plaque for Outstanding Export Performance ($3-Million Export Tower)

Award Plaque for Service Area
History




