
Product Planning
Market Research
-
Securement of item through Domestic and Overseas Market Research
-
Continuous Feasibility Study through Clinical Data
-
Securement of feasible drug
Approval Strategy
-
Preparation of specialized Documnet in Korea and compliance monitoring
-
Aggressive support for market exclusivity approval
-
Finding solution for difficult approval
Various API Supplier
-
Securement of feasible API through the domestic and overseas Partner
-
Securing new Supplier in the case of new Synthesis Planning
-
Making new partner with non-infringing ingredient
-
GMP level / Q11 supportable
Patent Strategy
-
Development of early launching items through non-infringing
-
Developing items for the patent-license linkage of Korea-US FTA
-
Securement of avoiding ingredient and formulation
Clinical Strategy
-
Preview of the needs for clinical trials in Korea
-
Preparation of clinical trials through CRO selection and IND approval
-
Systematic Clinical Trials through CRO management
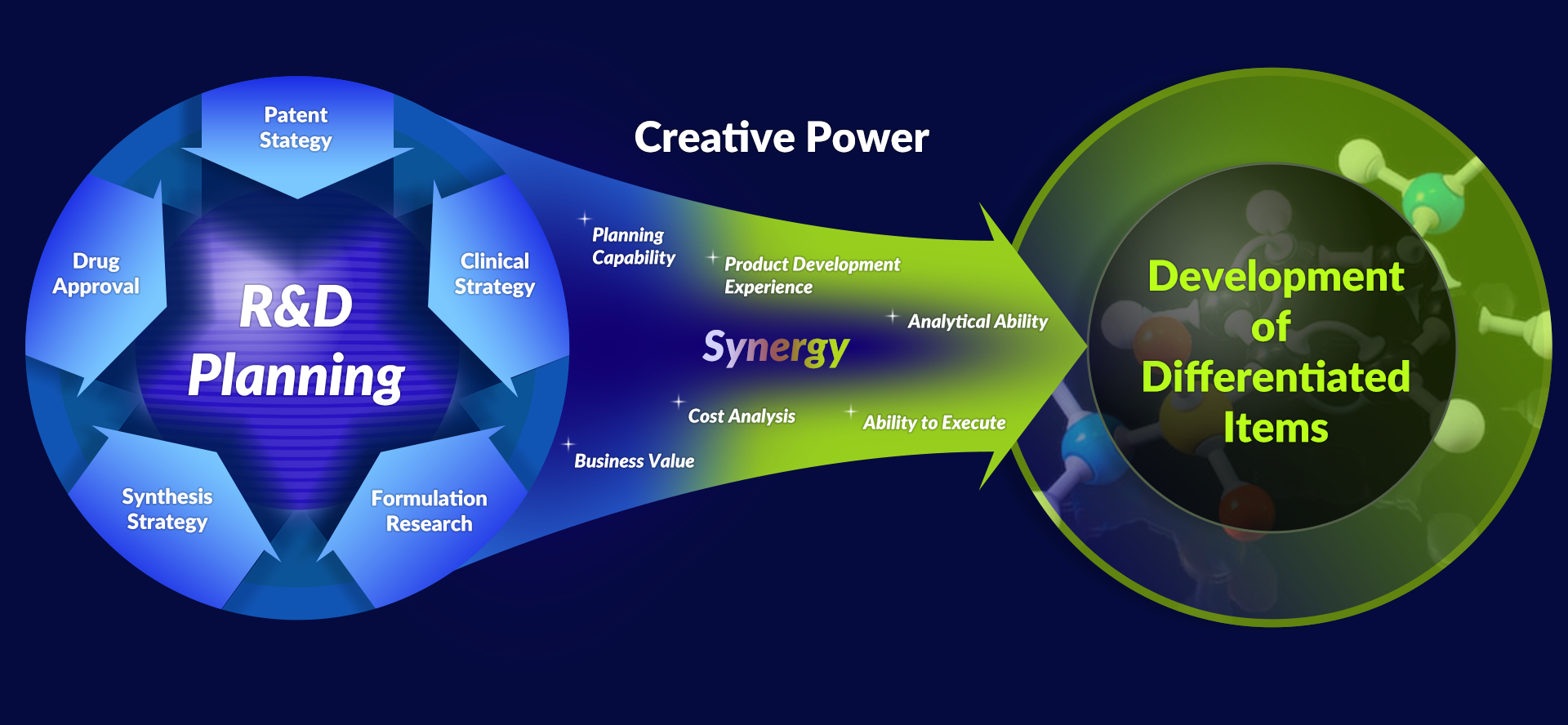
Creativity
-
Planning and Development Strategy for Newly Developed Item
-
New Item Development through Domestic and Overseas Partnership
-
Business diversification through New Business Model Development
Development of New Products
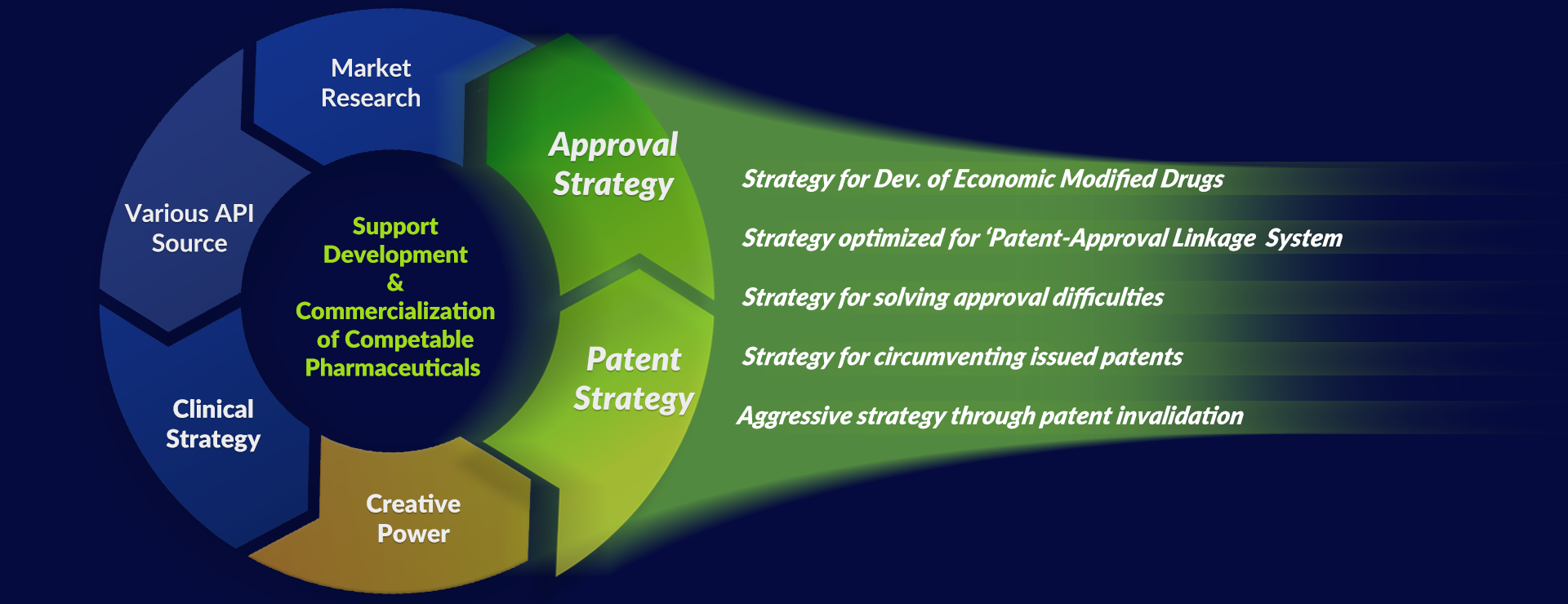
Differentiation of Product Planning
Based on our experiences in drug development and business success, Klasia supports the development of customized products for the market and customers through differentiated product planning on drug approval, patent, clinical trial, synthesis, and formulation research.
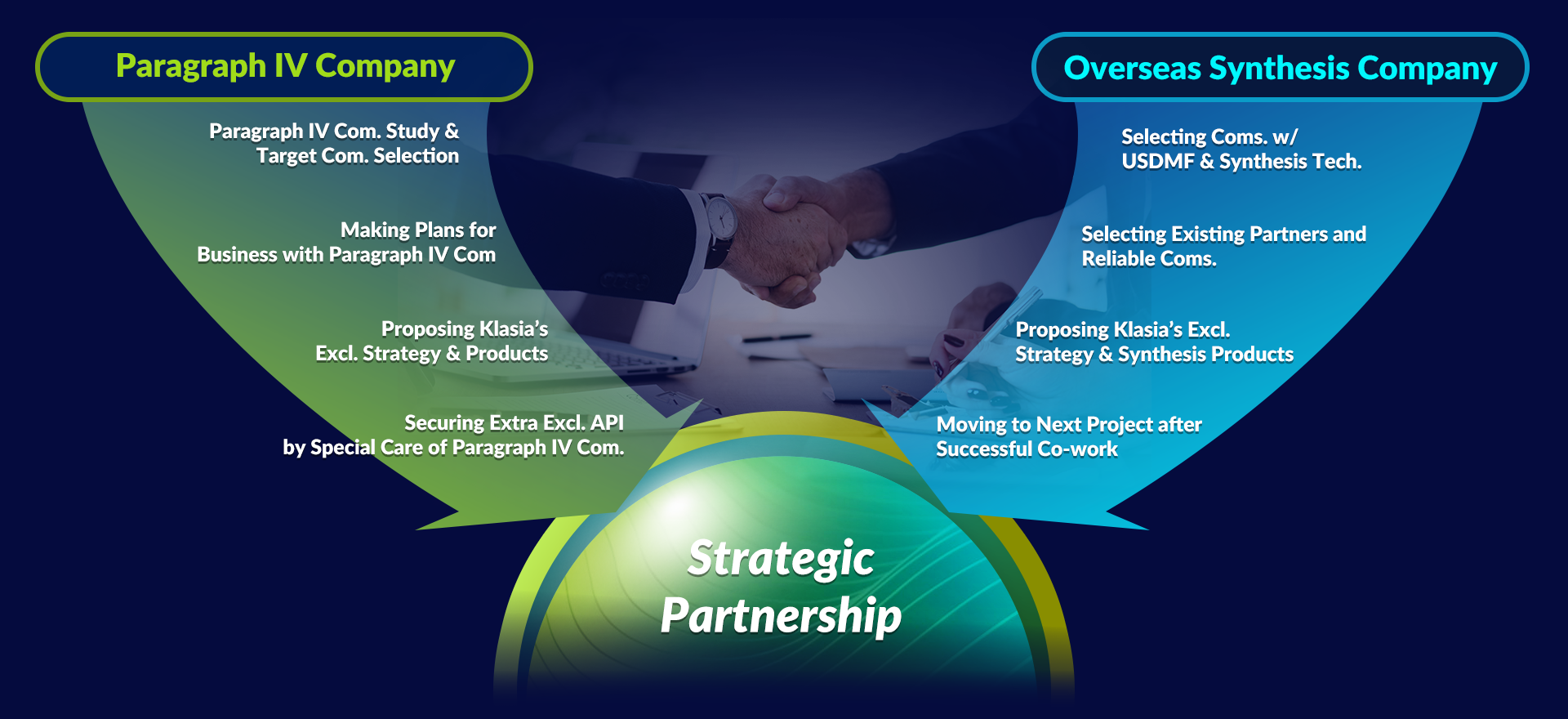
Differentiation through securing API
Klasia develops differentiated products from the stage of API through various types of raw material planning, and secures valuable API through R&D cooperation with many API suppliers and synthetic companies.

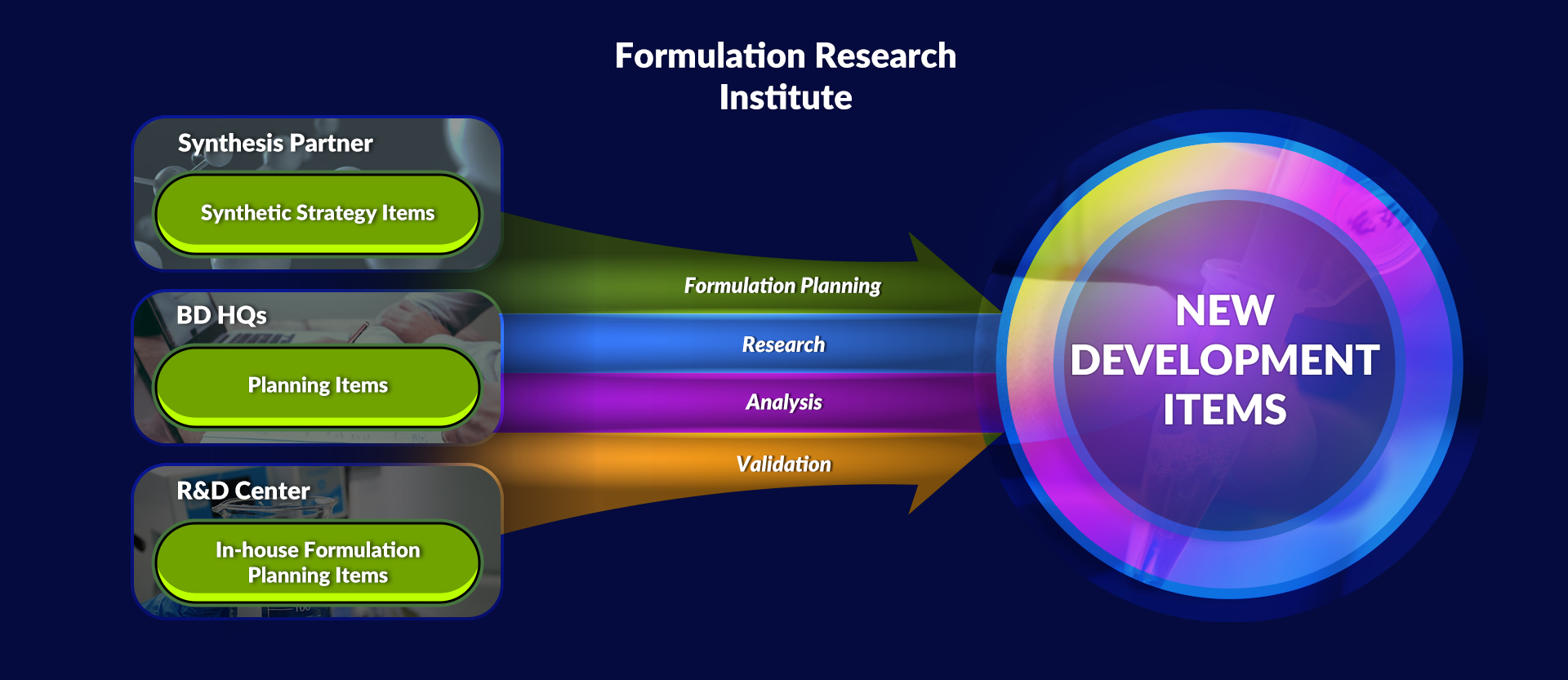
Differentiation through Formulation Research
Klasia operates a formulation research Institute with the best facility in Korea, and through this, it provides differentiated products that customers want through various studies.

Trading & Distribution
Klasia established infra in various countries through alliances. This enables us to develop the new business models. Through business experiences with many overseas domestic drug-related companies, we are creating various types of business, such as licensing finished products and R&D (synthesis, formulation, etc.) cooperation.


